The periodic table: one of the most iconic shapes. It is known from pole to pole, by scientist and non-scientist alike, and is as easily recognisable as the shape of Mickey Mouse. It adorns ties, t-shirts, and coffee mugs. It has found reference in art, and in pop culture. So what gave rise to this shape? Why is that that there are spaces and gaps? Are they placed there by accident or for artistic reasons? Well I am here to say: no, the shape of the periodic table was no accident. Each element has a deliberate and predictable space. Todays blog is about the meticulous observations and the man, who was one vote shy of a Nobel Prize, that resulted in one of the worlds most recognisable images: Dmitri Mendeleev.
Elements: these are the varying types of atoms that make up the Universe. Each element is a single type of atom. Many different types of elements come together to make molecules. Oxygen is an element-only has oxygen atoms; water is a molecule-has both oxygen and hydrogen atoms. By the middle of the 19th century, many elements were discovered by many scientists. Their atomic masses had been calculated, and many of their properties had been observed. But they had yet to be organised in some meaningful way. Though many had tried, none had succeeded.
Mendeleev, Chair of Chemistry at St. Petersburg University, began by writing the properties of each known element on a card. He then began arranging these cards in various ways, and it was not long before a pattern emerged. By arranging the elements in short rows according to atomic weight, placing the next row under the previous, columns emerged with elements that shared similar properties. Further, for this arrangement to work, gaps had to be left. Mendeleev believed these "gaps" represented elements yet to be discovered. Mendeleev therefore correctly predicted the elements gallium (discovered in 1875), scandium (discovered in 1879), and germanium (discovered in 1886). These elements, when discovered, easily fit into the gaps left for them. This table also showed that some of the atomic masses, such as those of gold and indium, had been incorrectly calculated. Mendeleev recalculated the masses and more accurate measurements would once again prove him correct.
In the 140 years that have passed since Mendeleev's first periodic table, the shape has changed greatly. However, the concept remains intact. Those patterns first observed in Mendeleev's table have resulted in the continuous, periodic arrangement of each newly discovered element.
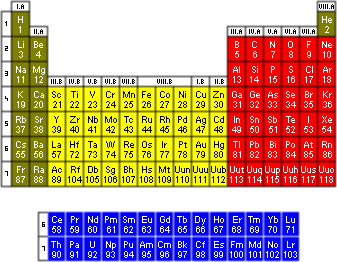
The modern periodic table consists of 18 groups (columns) compared with Mendeleev's 8. Each element is now arranged by its atomic number, rather than its mass. The atomic number is determined by the number of protons in the nucleus. Element 1 is hydrogen; hydrogen has one proton. Element 6 is my favourite element, carbon; carbon has six protons. Element 101, discovered in 1955, is mendelevium, as an homage to the author of the first periodic table; mendelevium has 101 protons. Each group consists of elements with similar properties. Each period (row) has elements increasing sequentially in atomic number.
One thing that is easily notable in the table is that there are distinct blocks, coloured differently in the table on the right. The green and red blocks consist of what are termed "the main group elements"; the yellow block is referred to as the "transition metals" and the blue block makes up the "rare earth metals" also called the "lanthanides and actinides". Each block has characteristic traits and give rise to interesting science. A lot of research has been devoted to studying periodic trends. It is important to acknowledge the blocks because they influence trends. For instance, going down a column in the main group block (example: group 14-carbon, silicon, germanium, tin, lead) we see that bond strengths decrease; however, going down the column in transition metals (example: group 8-iron, ruthenium, osmium) shows an increase in bond strength.
Take a look at group 14 again, we see that silicon falls right below carbon. Ever wonder why science fiction nerds make comments, jokes, references to silicon-based life forms? The answer falls in the periodic table. Because silicon is below carbon, it has similar properties. We are carbon-based life forms. The ability of carbon to catenate (meaning it bonds to itself in long chains of covalent bonds) well is what allows for life on this planet; silicon, being in the same group, also catenates well, theoretically meaning that it could form the backbone of life on a different planet.
It should be noted that there are some out there who feel that there are better, more accurate arrangements of the periodic table.
Mendeleev's table will forever be the first incarnation, quickly igniting revolution in chemistry.
References:
Petrucci, R. H.; Harwood, W. S.; Herring, F. G. General Chemistry 8th ed. 2002 Prentice-Hall Inc. Upper Saddle River, NJ.
Balchin, J. Quantum Leaps: 100 Scientists Who Changed the World 2010 Arcturus Publishing Limited., London.
Gray, T. The Elements 2009 Black Dog & Leventhal Publishers Inc., New York, NY.

Super interesting! Who knew? Well, chemists, I guess....but for the rest of us, very interesting!
ReplyDeleteLive Roulette | Casino site for the lucky club
ReplyDeleteCasino site for the lucky club, best odds, latest casino bonus, Casino site for the lucky club, best odds, latest casino bonus, lucky club website, luckyclub lucky club